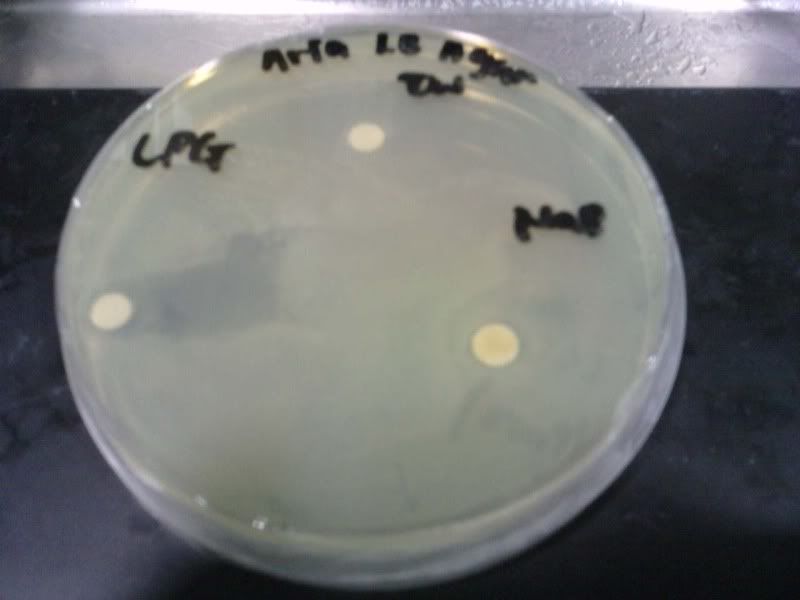
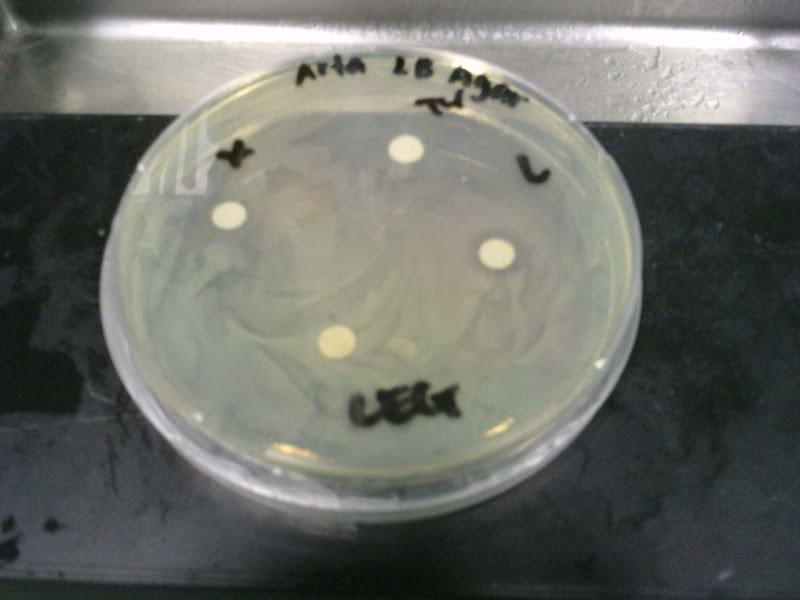
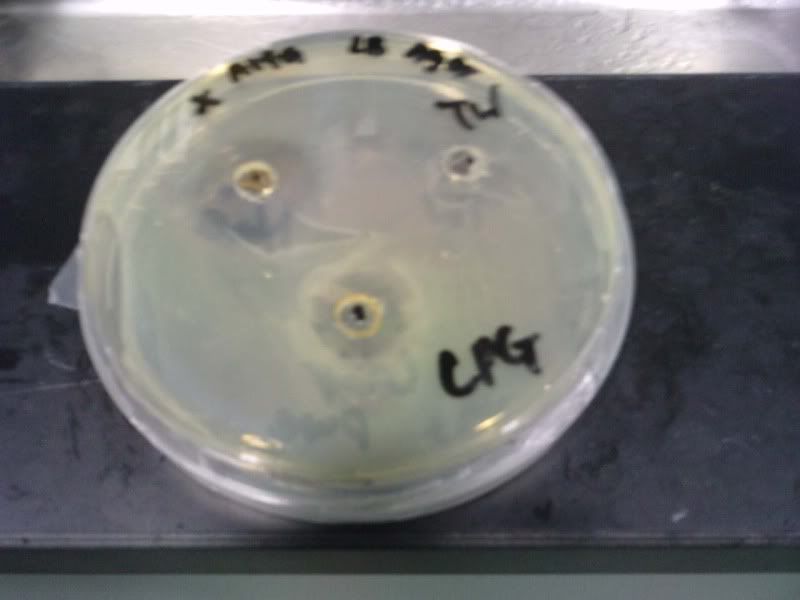
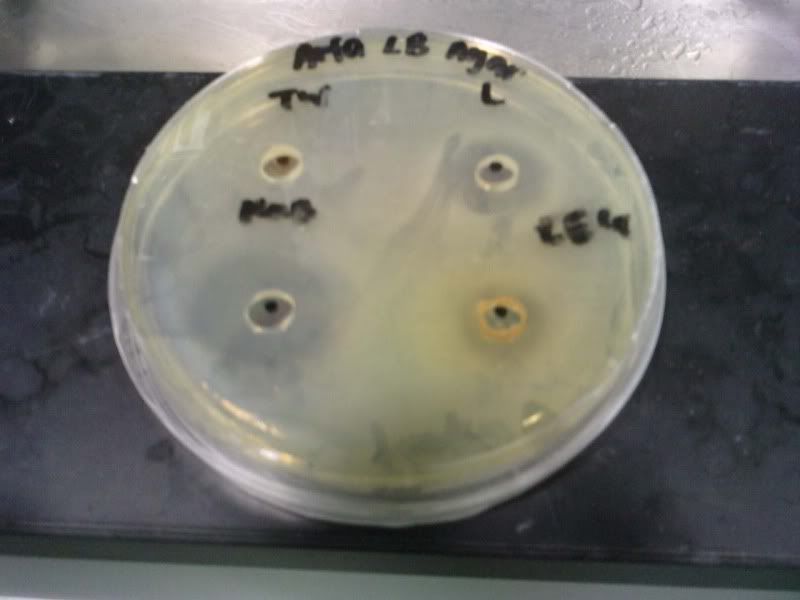
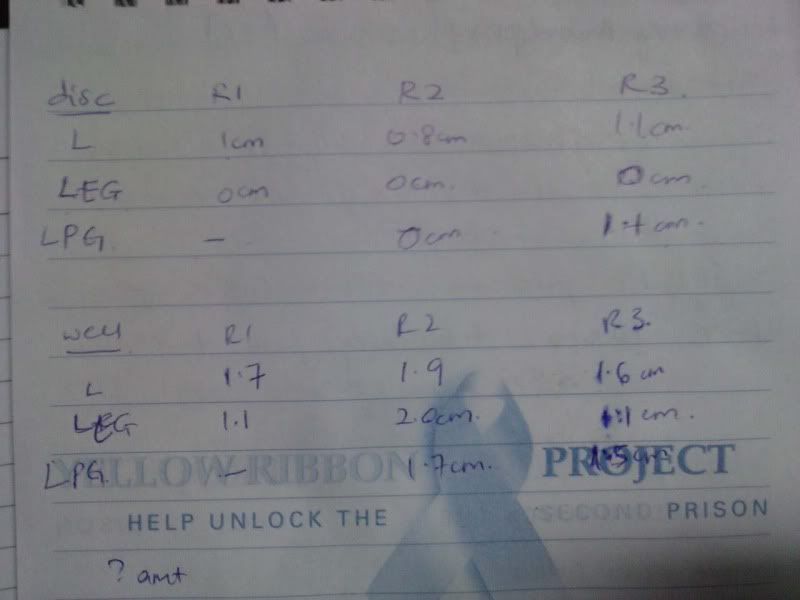
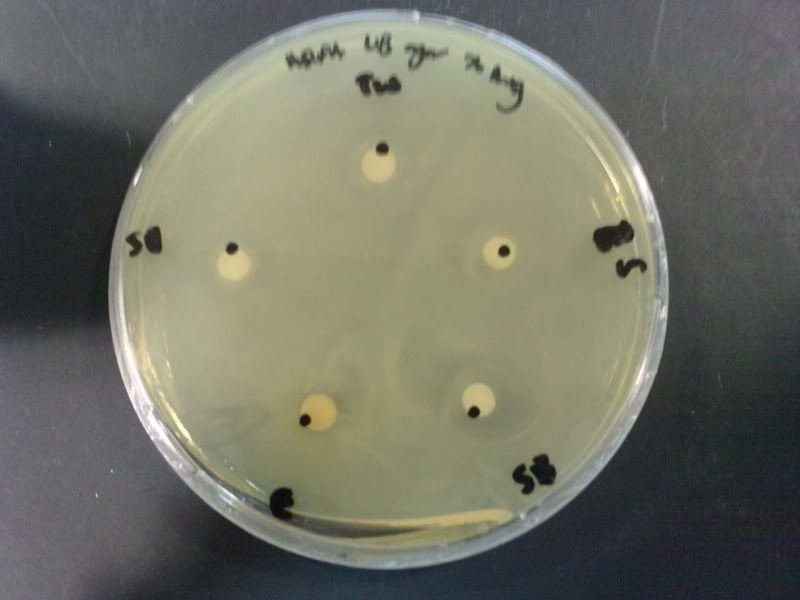
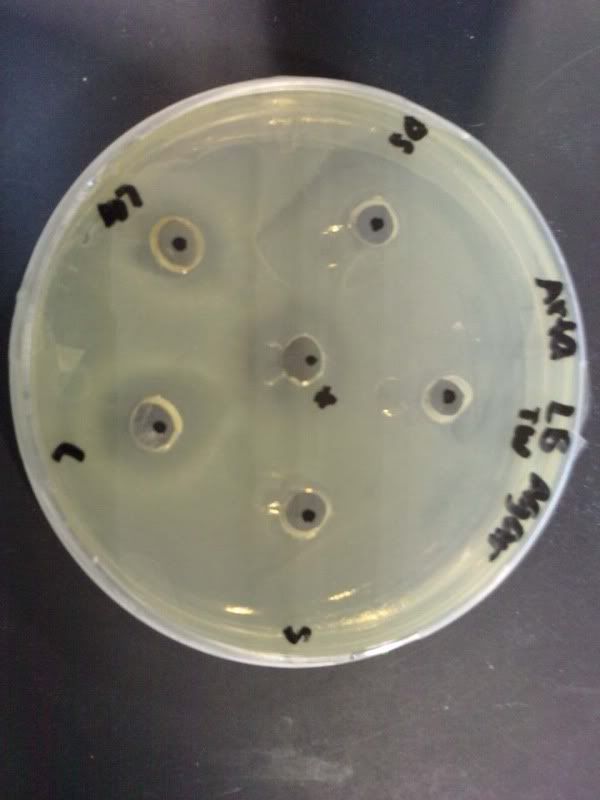
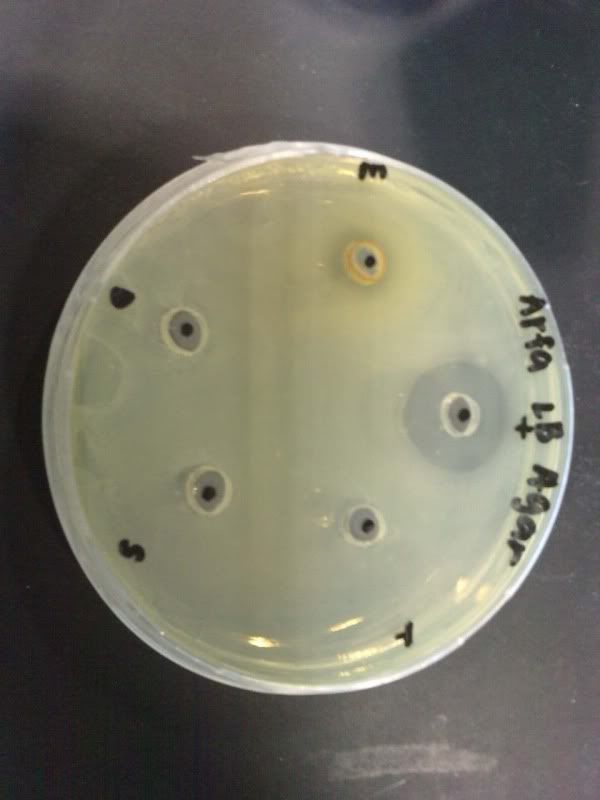
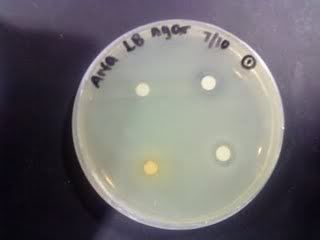
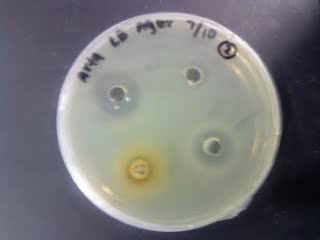
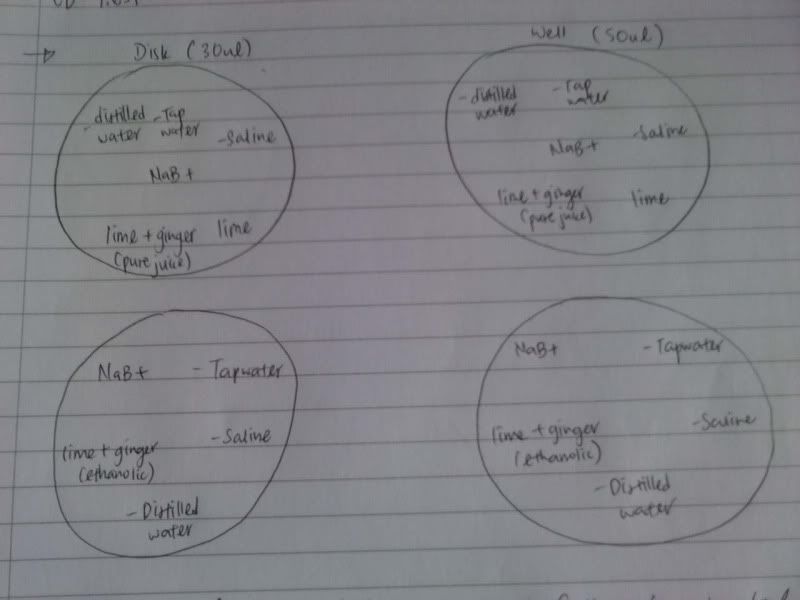
Time: 9.30 am – 4 pm
Present: Aina, Shila, Arfa
Absent: Pei Yi. Fadhilah
1. Check OD for E.Coli and the different concentration.
Pure Juice
A - 2.454
B - 1.110
C - 1.148
Ethanol
(1) - 1.197
(2) - 1.524
(3) - 2.675
Control (e.coli) - 1.394
I - 2.164
Time: 2.15pm - 9.45am (18+ hours)
2. Do serial dilution of Pure Juice, Ethanol and Control to 10^7.
Spread plate each set from 10^4 till 10^7.
Next plan of action is to check the serial dilution plates.
___________________________________________________________________
Result of all the serial dilution plates:




A4 – lot of colonies, uncountable.
A5 – confluent, not spreaded properly, lesser colonies than A4.
A6 – 36 colonies, lesser than A5.
A7 – clear of colonies.
the colonies decreased as the concentration decreased. confluent may be due to high od of 2.454.




B4 – confluent, uncountable
B5 – confluent, 100 colonies can be counted, not spreaded properly.
B6 – 32 colonies. lesser than B5.
B7 – 6 colonies. lesser than B6.
the colonies decreased as the concentration decreased. confluent may be due to high od of 1.110




C4 – lot of colonies, uncountable.
C5 – 300+ colonies
C6 – not spreaded properly
C7 – not spreaded properly
the colonies decreased as the concentration decreased. confluent may be due to high od of 1.148.
comparing A7, B7 and C7, as the extract increased, the bacteria growth increased too. this shows that pure juice of ginger does not have antimicrobial effect against e.coli. the optimal amount to inhibit bacteria could be around 100ul of pure juice extract.




14 – confluent, uncountable
15 – confluent, uncountable
16 – confluent, uncountable, not spreaded properly
17 – not spreaded properly, lesser than 17.
confluent could be due to not well spreaded. there might be contamination that affect the concentration.




24 – confluent
25 – confluent, not spreaded properly
26 – confluent, not spreaded properly, lesser than 25
27 – confluent, not spreaded properly, lesser than 26
confluent may be due to not well spreaded or contamination that affect the concentration.




34 – confluent
35 – confluent
36 – confluent
37 – confluent
too confluent could be due to high od of 2.675. there might be contamination that affect the concentration.
comparing 17, 27 and 37, 27 has lesser bacteria growth than 17. 37 has the most bacteria growth. the optimal amount of inhibiting bacteria could be around 300ml of ethanolic extract.




Cn4 – confluent
Cn5 – confluent
Cn6 – confluent, not spreaded properly
Cn7 – confluent
there might be contamination that affect the concentration. plates were not well spreaded. hence this is not a good control.
All the plates result don't tally with the od and therefore this serial dilution is not valid.