Present: (Aina, Shila, Arfa) -- arfa left at 1.42 pm
Time: 10 am - 3.30 pm
what do we gather
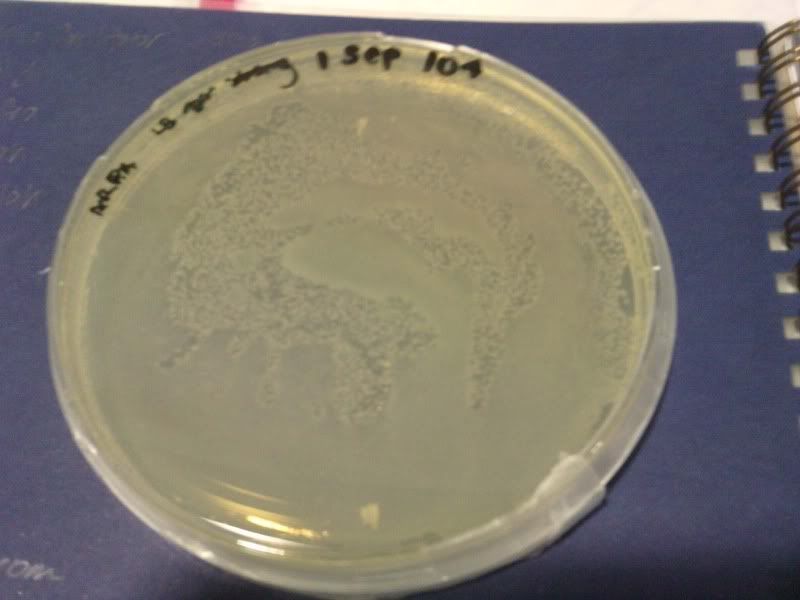
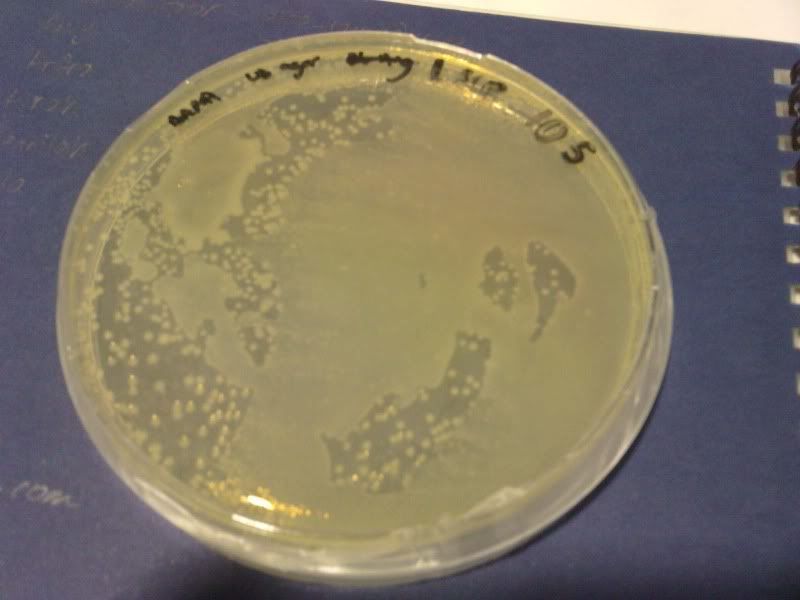
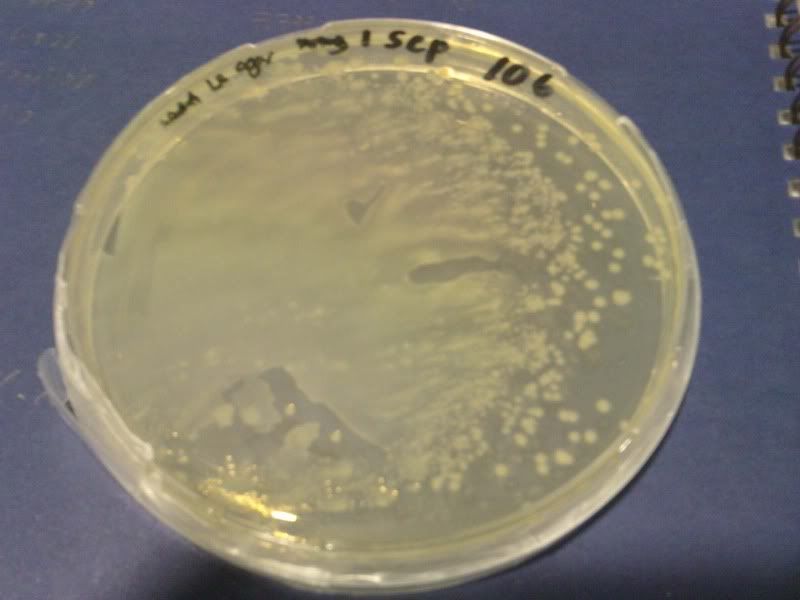
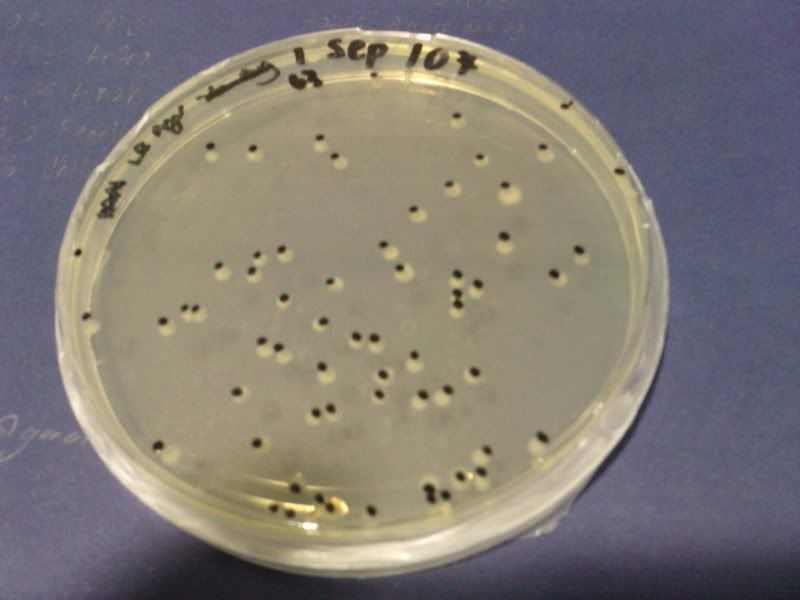
1) check od
time: 3.30 pm-10am
od: 2.080
2) measure the weight of the ethanol extract from the eppendorf tubes
A: 1.6825 - 1.0554 = 0.6271g
B: 1.7527 - 1.0500= 0.7027g
for A: 0.6271g were reconstitute with 1ml distilled water, vortex to dissolve
---> concentration: 0.6271g/ml
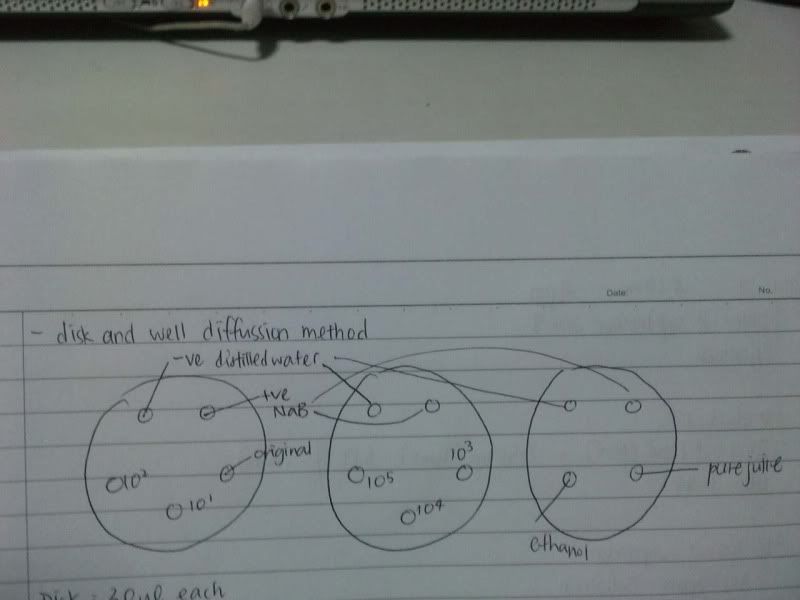
3) measure the zone of inhibition
*controls
- NaB 1.8
- E 1.3
*well
NaB, W, 10*3, 10*4, 10*5 ( NaB 1.6)
*disc
NaB, W, 10*3, 10*4, 10*5 ( NaB 1.7)
*well
NaB, W, 0, 10*1, 10*2 ( NaB 1.8)
*disc
NaB, W, 0, 10*1, 10*2 ( NaB 1.7)
*well
W, NaB, E, PJ ( NaB 1.8)
*well
W, NaB, E, PJ ( NaB 1.5)


4) do agar disc and well diffusion ( incubate at 1.45 pm )
Well :
- NaB, W, PJ
- NaB, W, E
-NaB, W, HW
Disc: ( 25 ul 1st, let it dry, another 25 ul added )
- NaB, W, PJ
- NaB, W, E
-NaB, W, HW
5) innoculate different concentration of PJ , Ethanol and control (incubate at 3.15pm )
Pure juice
A- 5ml broth & 50ul e.coli & 200 ul extract
B-5ml broth & 50ul e.coli & 300 ul extract
C- 5ml broth & 50ul e.coli & 400 ul extract
ethanol
1-5ml broth & 50ul e.coli & 200 ul extract
2-5ml broth & 50ul e.coli & 300 ul extract
3-5ml broth & 50ul e.coli & 400 ul extract
control
5ml broth & 50 ul e.coli
6) innoculate 10ml broth and 100 ul e.coli (I) (incubate at 3.15 pm)
7) grind ginger using food processor and measure 0.1076g into agar well with NaB and W.
(incubate at 2.30 pm )
next plan of action:
check OD for the e.coli and the different concentration of PJ and ethanol
check zone of inhibition
do serial dilution
what do we gather
1) check od
time: 3.30 pm-10am
od: 2.080
2) measure the weight of the ethanol extract from the eppendorf tubes
A: 1.6825 - 1.0554 = 0.6271g
B: 1.7527 - 1.0500= 0.7027g
for A: 0.6271g were reconstitute with 1ml distilled water, vortex to dissolve
---> concentration: 0.6271g/ml
3) measure the zone of inhibition
*controls
- NaB 1.8
- E 1.3
*well
NaB, W, 10*3, 10*4, 10*5 ( NaB 1.6)
*disc
NaB, W, 10*3, 10*4, 10*5 ( NaB 1.7)
*well
NaB, W, 0, 10*1, 10*2 ( NaB 1.8)
*disc
NaB, W, 0, 10*1, 10*2 ( NaB 1.7)
*well
W, NaB, E, PJ ( NaB 1.8)
*well
W, NaB, E, PJ ( NaB 1.5)


4) do agar disc and well diffusion ( incubate at 1.45 pm )
Well :
- NaB, W, PJ
- NaB, W, E
-NaB, W, HW
Disc: ( 25 ul 1st, let it dry, another 25 ul added )
- NaB, W, PJ
- NaB, W, E
-NaB, W, HW
5) innoculate different concentration of PJ , Ethanol and control (incubate at 3.15pm )
Pure juice
A- 5ml broth & 50ul e.coli & 200 ul extract
B-5ml broth & 50ul e.coli & 300 ul extract
C- 5ml broth & 50ul e.coli & 400 ul extract
ethanol
1-5ml broth & 50ul e.coli & 200 ul extract
2-5ml broth & 50ul e.coli & 300 ul extract
3-5ml broth & 50ul e.coli & 400 ul extract
control
5ml broth & 50 ul e.coli
6) innoculate 10ml broth and 100 ul e.coli (I) (incubate at 3.15 pm)
7) grind ginger using food processor and measure 0.1076g into agar well with NaB and W.
(incubate at 2.30 pm )
next plan of action:
check OD for the e.coli and the different concentration of PJ and ethanol
check zone of inhibition
do serial dilution